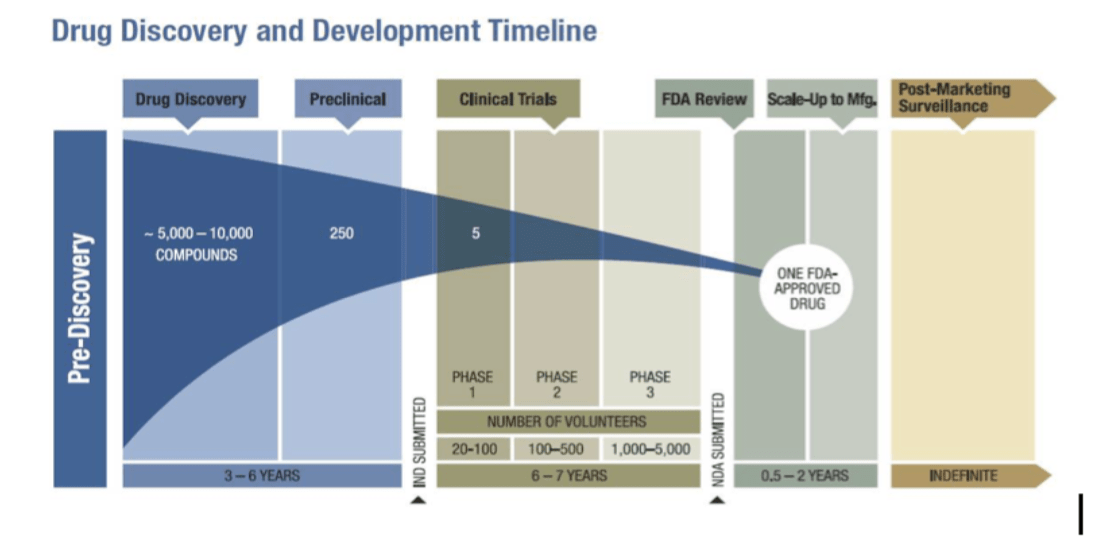
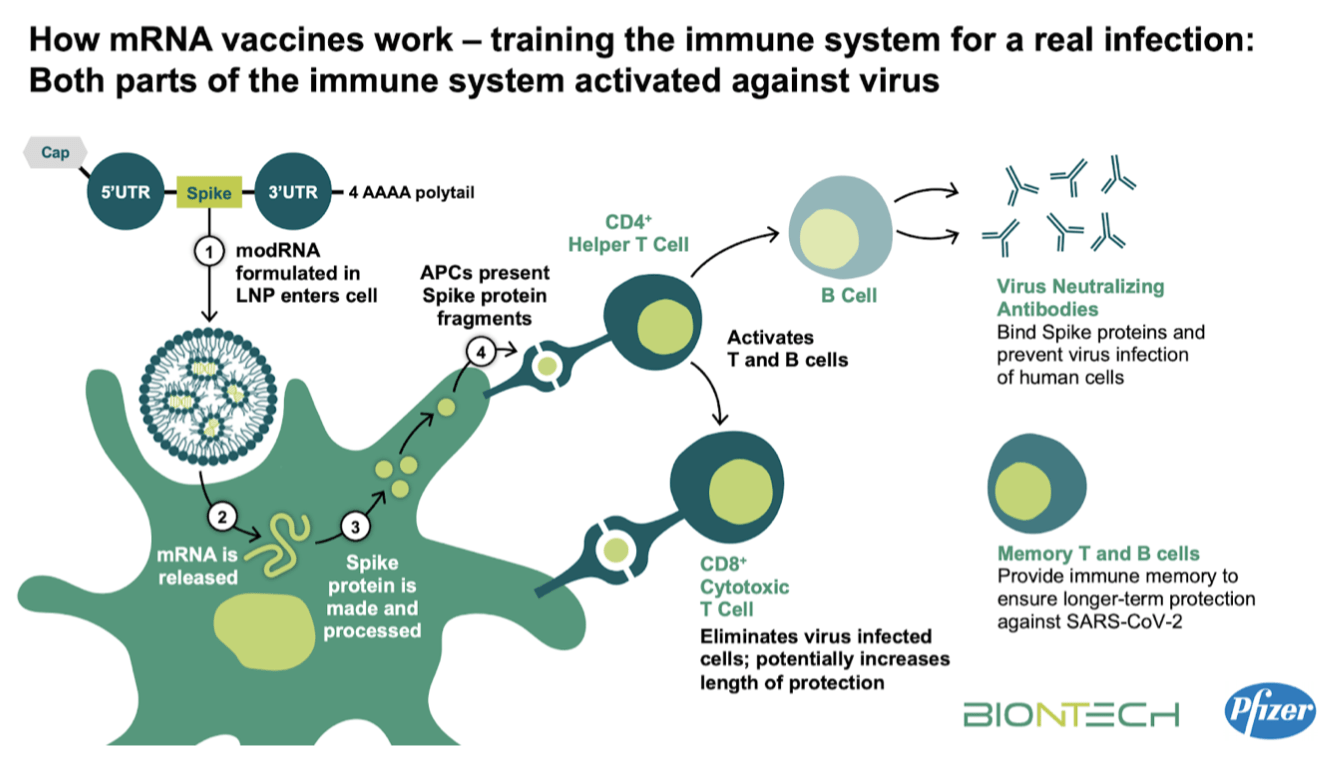
Do the vaccines actually work?
Short answer: Yes they do and the results are available in the detailed Phase III trial data produced by the three companies in the last two months.
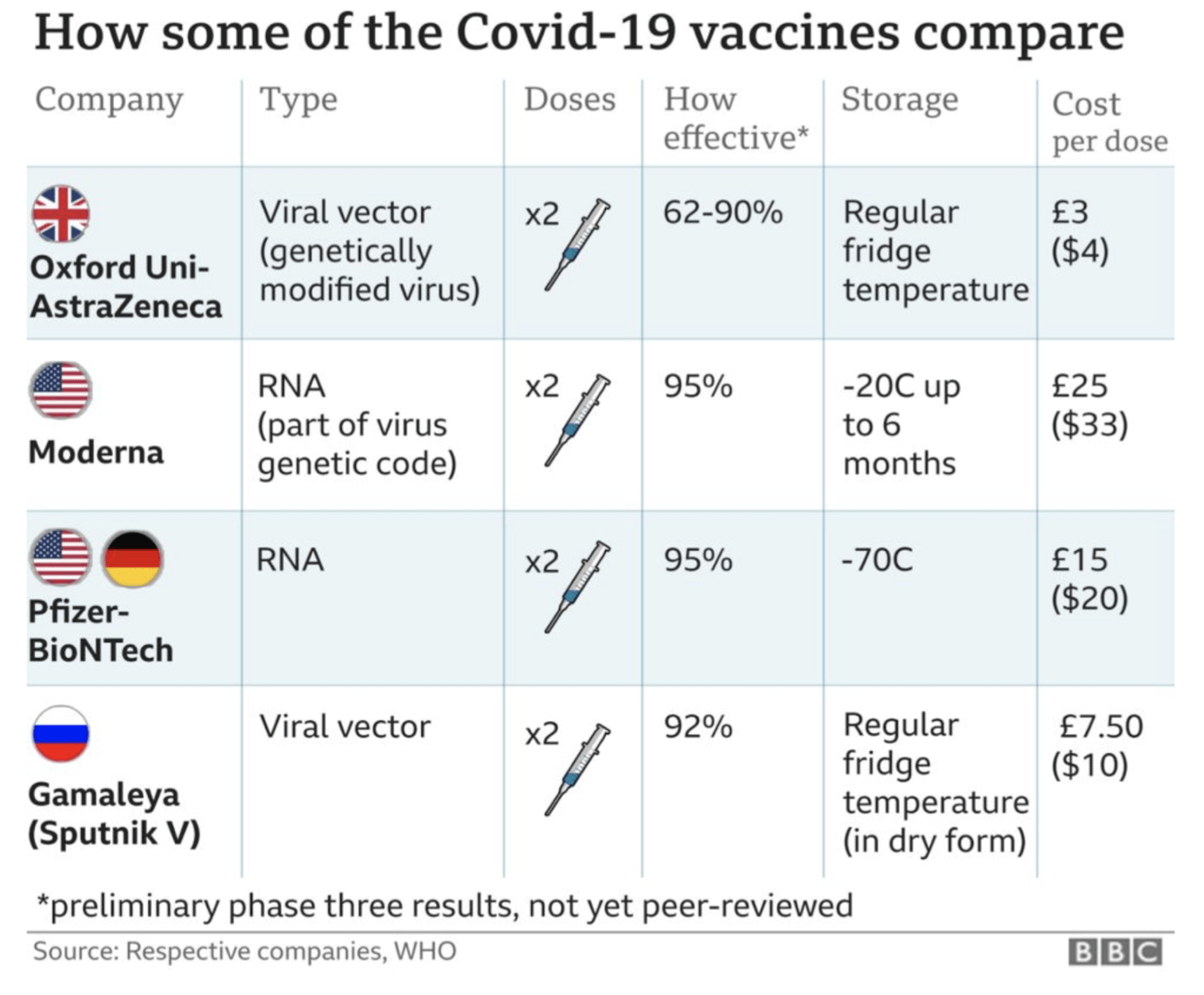
Long answer: Moderna & Pfizer/BioNtech vaccines have similar efficacy whilst AstraZeneca has less efficacy but pricing & storage/effective delivery becomes an important factor in deciding which vaccine is best for which country.
Click here for our previous blog on Busting the Vaccine Myths
Pfizer/BioNTech
A two dose vaccine with the second dose to be administered 28 days after the first dose. Study data showed a 95% efficacy rate 7 days after the second dose with efficacy in adults over age of 65 at an excellent 94%. The final analysis was to be done at 164 case mark but instead they were able to surpass and hit the 170 case mark (due to the prevalence of COVID19). 170 people enrolled in the trial contracted COVID19 – when called back, it turns out 162 were from the placebo arm compared to 8 in the vaccine arm.
Approved by the FDA and the EU for emergency use, Pfizer/BioNtech vaccine has been deployed since early December in countries across the world. Due to the sensitivity of the mRNA vaccine compound, the vaccine requires storage at -70C to prevent denaturing of the key compound and rendering the vaccine vials useless. As such, cold storage supply chains are being developed around the world to safeguard against denaturation in transit. Given the freezing temperatures required, the costs associated with deep freeze supply chain equipment and the precision with which the vaccine must be administered post vial opening, this particular vaccine deployment may be difficult to execute in south asian countries such as Sri Lanka.
Moderna
Another two dose vaccine with the second dose administered 28 days after the first. Data showed a 94.1% efficacy rate 7 days after the second dose. Final analysis happened at the 196 case mark with 185 cases in the placebo group and 11 in the vaccine group. Interestingly, the analysis included 30 severe cases of COVID19 – all of which were in the placebo group and none in the vaccine group heralding this vaccine as better at preventing severe outcomes of the disease.
The vaccine can be stored at -20C (normal refrigeration temperatures) for up to six months making them more friendlier to tropical countries and eliminating the heavy investment into logistics that may be required of Pfizer/BioNtech. Given the novel nature of the mRNA vaccines, those who administer the vaccine need to work in a timely and efficient manner to avoid wastage and may require upskilling of existing healthcare staff to ensure the same.

AstraZeneca
Earlier created to be a two dose vaccine, a mistake during one part of the trial resulted in a surprising finding that administering half a dose first and then a full dose second resulted in 90% efficacy vs. 62% efficacy in those getting the two full doses. However, only 2,700 persons were administered the “mistaken” dosage and this requires further study. This leaves AstraZeneca as the vaccine candidate with the least efficacy when compared to the two mRNA vaccine options. Results were based on a 131 case mark with 30 in the control group and 11 in the vaccine group contracting the virus.
AstraZeneca remains the cheapest vaccine at GBP 3/dose and can be stored at room temperature (like most other existing vaccines) allowing health workers to work in a familiar setting.
If you are questioning which vaccine should you take: given what is known of COVID19 (its rapid spread, its attack on the respiratory and vascular systems of the body, long COVID afflicting patients six months post initial recovery and the fatality rate), all of these vaccines appear as attractive alternate options.
- https://www.profil.com/knowledge-center/trial-stages
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6226120/
- https://investors.biontech.de/static-files/53f0968a-279b-4f82-a2fc-d67dcb6e4e91
- https://www.modernatx.com/modernas-work-potential-vaccine-against-covid-19
- https://www.health.harvard.edu/blog/why-are-mrna-vaccines-so-exciting-2020121021599
- https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-conclude-phase-3-study-covid-19-vaccine
- https://www.livemint.com/science/health/oxford-astrazeneca-publish-final-stage-results-covid-vaccine-70-effective-shows-lancet-data-11607444483396.html
Similar Articles...

Let’s talk flu, its prevention and home remedies.
Boo-ger season is here! Let’s begin by defining flu (short term for influenza) because it’s usually misunderstood as fever or cold. Flu is a common

What’s The Deal With COVID19 Boosters?
As Sri Lanka rolls out its COVID19 booster program, we break down the answers to your most pressing questions. Firstly, what is a booster? A

Back to School – A Battle Between Education and COVID-19
Back to School – A Battle Between Education and COVID-19 Students are finally returning to school. But as parents, many are worried about COVID-19 safety.